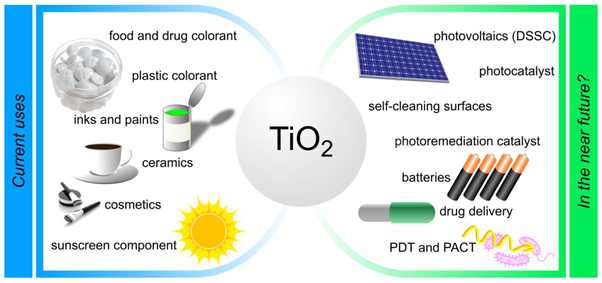
Titanium Dioxide (Tio2) is used in loads of industrial and consumer products like paints, inks, paper, plastics, textiles, ceramics, construction materials, cosmetics, food and pharmaceuticals.
Titanium Dioxide presently classified as non-hazardous has been proposed for re-classification as carcinogenic (cancer-causing) substance by the EU Commission per EU Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. See reclassification detail in 14th ATP (Adaptation to Technical Progress) to CLP Regulation which applies apply from October 2021.
Tio2 ,as a dust when inhaled, has been classified by the International Agency for Research on Cancer (IARC) as an IARC Group 2B carcinogen. Their findings on respiratory toxicity are based on high concentrations of pigment-grade (powdered) ultrafine Tio2 dust causing respiratory tract (lung) cancer in rats after inhalation.
With that said, it would require a new warning phrase:
Liquid mixtures EUH211 ‘Warning! Hazardous repairable droplets may be formed when sprayed. Do not breath spray or mist.’
Solid mixtures EUH212 ‘Warning!’
The European Council of the Paint, Printing Ink and Artists’ Colours Industry (Cepe) and TDMA are working closely to assess the impact of the classification, however consequences include:
- many paints no longer being available;
- twice as much paint used to achieve comparable opacity;
- building rubble, plaster and wallpaper containing titanium dioxide become hazardous waste; and
- eco labels cease to apply to many consumer products.
ProductVision Regulatory Compliance (RC) enables users to create and maintain Material Safety Data Sheets (SDSs) and other regulatory documents. The reclassification of Titanium Dioxide is quickly and easily captured in RC.
The ProductVision® product development software is a product lifecycle management (PLM) system that manages all aspects of the product development process. Suitable for industries including paint & coatings, personal care and food & beverage, ProductVision’s comprehensive list of modules includes Formula Management, Regulatory, GHS, Project Management, Document Management, Ingredient Labelling, Guidelines & Restrictions, Sample Tracking, Testing and Workflow. We have your complete development process covered no matter what your industry.
Further reading and sources:
https://pubmed.ncbi.nlm.nih.gov/20156837/
BCF COVERED Spring 2020
BCF Digest 120 March 2020
